PHOMED’s Approach to High-Stability EVLA Laser Technology
The Application of Surgical Lasers in EVLA Therapy and PHOMED’s Approach to Device Development
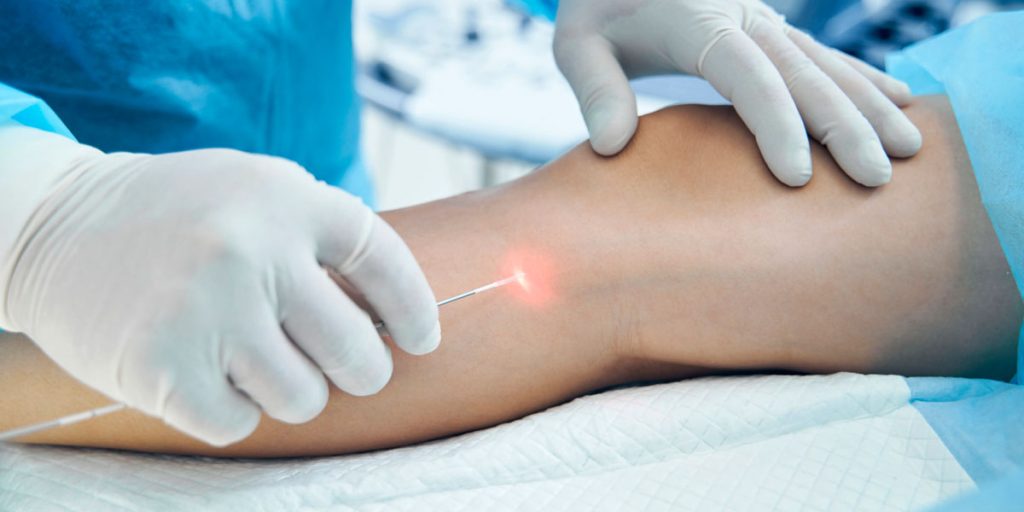
Endovenous Laser Ablation (EVLA) has become an established minimally invasive option for treating venous reflux. While the clinical technique receives most of the attention, the underlying success of EVLA depends largely on the precision, stability, and reliability of the laser equipment used during the procedure.
As a company dedicated to the research, development, and manufacturing of surgical laser systems, PHOMED approaches EVLA not only from a therapeutic perspective but from an engineering and technology foundation. Our work focuses on understanding the physical principles of light–tissue interaction and transforming them into safe, efficient, and highly stable medical devices.
01. Technical Requirements of EVLA: An Engineering Perspective
Although EVLA is performed in a clinical environment, the treatment outcome is directly determined by how well the laser system delivers controlled optical energy. A device used for EVLA must meet several stringent engineering requirements:
1. Output Stability Under Dynamic Conditions
During the procedure, the fiber is continuously withdrawn inside the vein. The laser must deliver a stable, predictable output even as load conditions change, without surges, drops, or thermal drift.
2. Wavelength Selection Based on Tissue Absorption
Different wavelengths interact with blood and vessel walls in different ways. A well-designed system must ensure high spectral purity, consistent wavelength accuracy, and optimal conversion efficiency according to the selected absorption target.
3. Precise Energy Control
EVLA relies heavily on linear endovenous energy density (LEED). The laser equipment must provide precise control over power, pulse characteristics, and energy delivery to support consistent clinical results.
4. Safe and Durable Fiber Transmission
Since the optical fiber is the interface between the device and the body, its thermal stability, mechanical durability, and reliability are essential to safe operation.
These requirements highlight why the effectiveness of EVLA depends not only on surgical skill but on the engineering quality of the device itself.
02. PHOMED’s R&D Philosophy: Building a More Predictable Laser Platform
PHOMED’s product development for EVLA systems is guided by an engineering-first philosophy:
a surgical laser should not simply emit light—it must deliver the right energy, in a stable and fully controllable manner, throughout the entire procedure.
Our R&D focuses on several core areas:
1. Optical Stability as a Foundational Principle
We conduct comprehensive evaluations of optical stability that include:
- long-duration continuous-output testing,
- thermal drift and load-response analysis,
- batch-to-batch optical consistency,
- wavelength accuracy and beam-quality verification.
This ensures that every device maintains clinical performance across different operating conditions.
2. Optimized Wavelengths and Fine-Tuned Energy Control
For the wavelengths commonly used in EVLA (e.g., 980 nm and 1470 nm), PHOMED has established specialized control algorithms to improve:
- drive-current stability,
- transient response at power changes,
- real-time feedback loops for energy regulation.
These improvements help physicians deliver predictable thermal effects regardless of vein diameter or pull-back speed.
3. Enhanced Fiber-End Safety and Mechanical Reliability
PHOMED continually refines its fiber designs with attention to:
- thermal endurance at the distal tip,
- uniform optical energy dispersion,
- reinforced mechanical structure,
- user-friendly connector engineering.
Through iterative design and testing, we aim to reduce hot-spot formation and strengthen resistance to bend-related damage.
4. Internal Heat-Transfer and Tissue-Interaction Modeling
To support development decisions, PHOMED maintains an in-house simulation and testing environment that includes:
- heat-transfer profiles in tissue-equivalent materials,
- comparisons of energy delivery at various pull-back speeds,
- mapping of temperature gradients under different wavelength-power combinations.
This structured validation process helps us refine device parameters before they reach clinical use.
03. Manufacturing Excellence: Consistency From Component to Final System
A high-quality surgical laser is the result of precise engineering combined with strict manufacturing standards. PHOMED ensures reliability through:
- individual calibration for each laser module,
- fiber-end inspection and loss testing for every batch,
- functional verification at the subsystem and full-system levels,
- continuous monitoring of optical output throughout production.
This level of process control ensures the reproducibility that physicians and hospitals rely on.
04. Regulatory and Quality Systems
PHOMED operates under established medical-device quality management frameworks such as ISO 13485, implementing structured control from R&D to mass production. For EVLA-related products, we conduct testing in areas including:
- laser safety and electrical safety,
- electromagnetic compatibility (EMC),
- mechanical endurance of fibers and connectors,
- material biocompatibility where applicable.
Our commitment is to create devices supported by measurable, verifiable, and transparent engineering data—not assumptions or unvalidated claims.
05. PHOMED’s Mission: Safer Energy, Better Control, Greater Confidence
EVLA has become a routine procedure in modern vascular care, but its safety and effectiveness remain closely tied to the performance of the laser equipment used. PHOMED strives to elevate this standard by providing:
- stable optical output,
- precise and predictable energy control,
- reliable fiber-optic delivery,
- transparent and verifiable device parameters,
- systems engineered around clinical workflow,
- consistent quality at scale.
We believe that a well-designed laser device should reduce variability, enhance confidence, and support better patient outcomes—not add complexity.
Conclusion
EVLA is built on controlled photothermal interaction, and PHOMED’s mission is to provide the technology that makes this interaction safer, more predictable, and more reliable. Our focus on optical engineering, control systems, fiber-optic safety, and rigorous validation forms the foundation for the next generation of surgical laser solutions.
We will continue to invest in research and development across wavelengths, energy control, optical delivery, and intelligent monitoring systems—bringing forward devices that meet the evolving needs of clinicians while upholding the highest engineering standards.